Customized Disposable Plasma Surgical Electrode CDMO Service Provider
In the ever-evolving landscape of medical technology, finding a reliable and innovative partner for the development and production of medical devices is crucial. At SmartVein Medical Technology, we stand at the forefront of providing customized disposable plasma surgical electrode Contract Development and Manufacturing Organization (CDMO)services, tailored to meet the unique needs of our clients. Our expertise spans across the entire product lifecycle, from conceptualization to commercialization, ensuring that every step of the process is meticulously executed with precision and innovation.

Comprehensive Service Offering
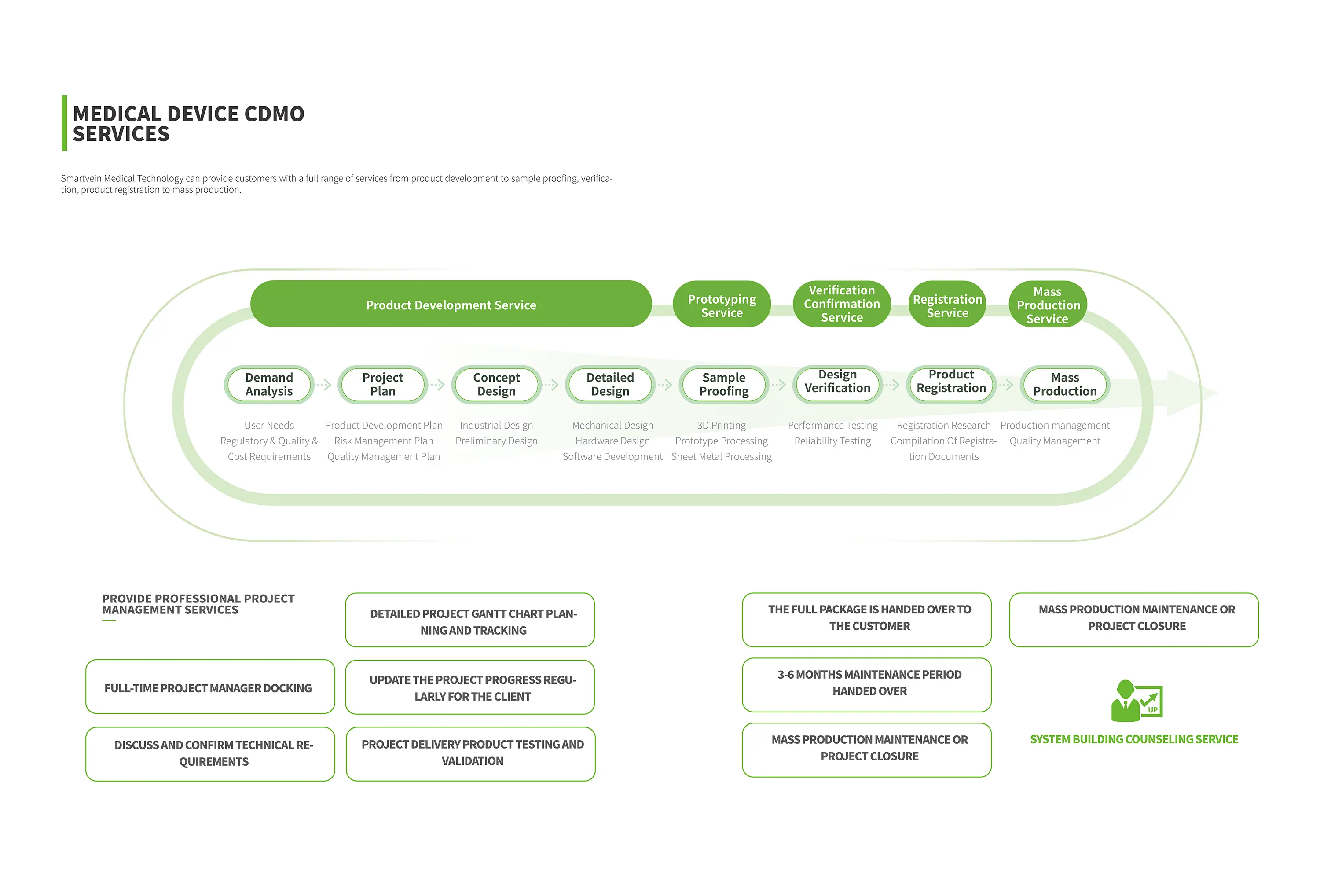
SmartVein Medical Technology's core strength lies in our ability to offer a full range of services that cater to every stage of a medical device's journey. We understand that each client has distinct requirements, and our flexible approach allows us to tailor our services accordingly.
1. Product Development
Our journey with clients begins at the product development stage. SmartVein's team of seasoned engineers and designers collaborates closely with clients to understand their vision and translate it into a feasible, innovative product. We leverage cutting-edge technology and rigorous testing protocols to ensure that the initial design meets all regulatory requirements and performs optimally under various conditions.
2. Sample Proofing and Verification
Once the initial design is finalized, we proceed to sample proofing. This stage involves creating prototypes that undergo rigorous testing to validate their functionality, durability, and safety. Our in-house testing facilities are equipped with state-of-the-art equipment that simulates real-world conditions, ensuring that our plasma surgical electrodes are ready to perform flawlessly in clinical settings.
3. Product Registration
Navigating the complex regulatory landscape of medical devices can be daunting. SmartVein Medical Technology offers comprehensive product registration services that simplify the process for our clients. Our regulatory affairs experts stay abreast of the latest changes in global medical device regulations, ensuring that our clients' products comply with all necessary standards and receive timely approval for market entry.
4. Mass Production
With product registration secured, we transition seamlessly into mass production. Our manufacturing facilities are designed to meet the highest standards of quality and efficiency. We employ advanced automation and lean manufacturing principles to ensure consistent, high-quality output while minimizing waste and downtime. Our commitment to excellence extends to every aspect of production, from raw material sourcing to final packaging, ensuring that our clients receive only the best.
Conclusion
SmartVein Medical Technology is the leading provider of customized disposable plasma surgical electrode CDMO services, offering a full range of services from product development to mass production. Our commitment to innovation, quality, and customer satisfaction sets us apart as a trusted partner in the medical device industry. With our expertise and dedication, we are confident that we can help our clients bring their vision to life, creating safe, effective, and innovative medical devices that make a difference in the lives of patients worldwide.
www.smartveingroups.net
SmartVein Medical Technology